A Clinical Trial, or Experimentation, is a medical research aimed at:
- Discovering or verifying the effects of a new clinical approach (preventive, therapeutic, or even diagnostic).
- Identifying any adverse reactions to one or more experimental drugs or medical devices.
- Studying their absorption, distribution, metabolism, and elimination, with the aim of ensuring their safety and/or effectiveness, as well as other scientific and non-scientific elements.
The total number of active clinical trials in the triennium 2021-2023 within the Department is N.365.
The graphs below show the percentages related to the type and phases of experimentation.
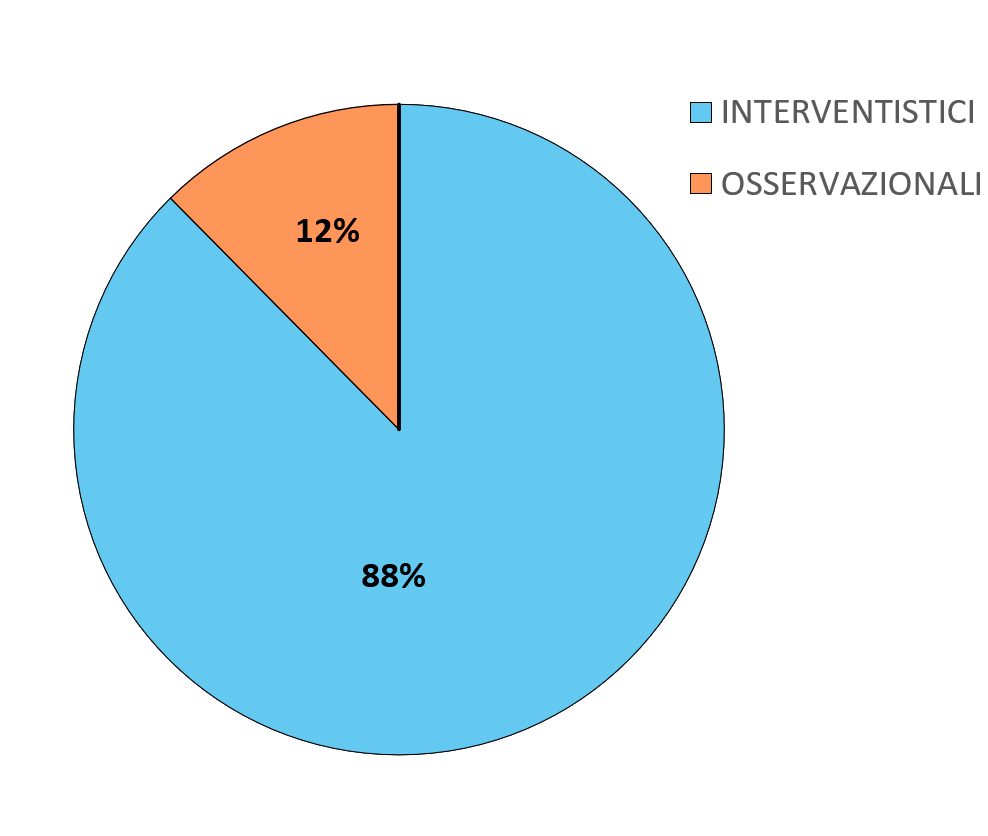
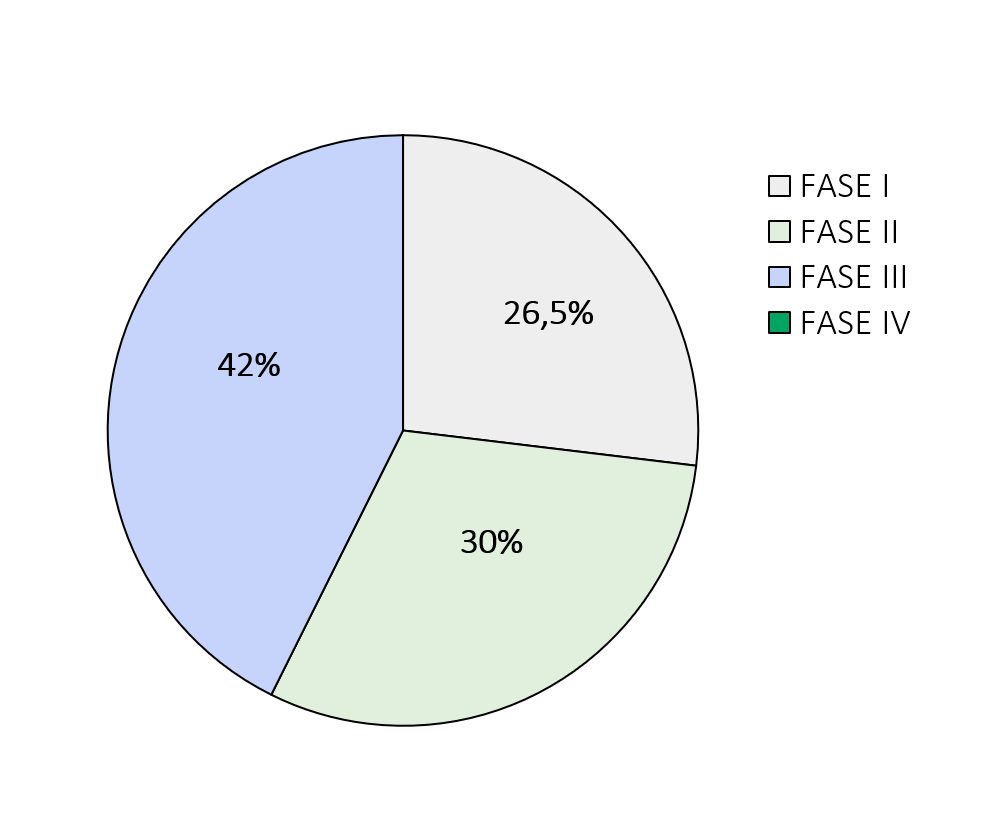
The phases of a clinical trial are:
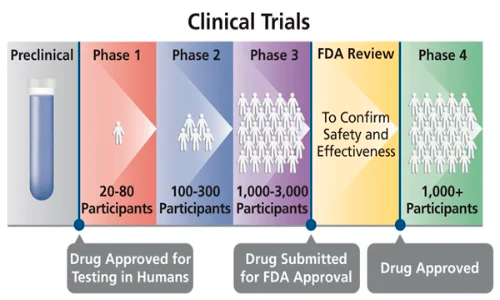
(https://www.thedifferentgroup.com/2020/11/20/studio-clinico/)
Clinical trials are categorized into:
- Profit, promoted by the pharmaceutical industry for profit purposes, whose results become the property of the pharmaceutical industry and can be used for drug industrial development, regulatory purposes, or commercial purposes;
- Non profit, aimed at improving clinical practice as an integral part of healthcare and not for industrial purposes.
Considering the implications for scientific research, professors and researchers in the medical field affiliated with the National Health Service may have the role of Principal Investigator in profit and non-profit clinical trials conducted at the Complex Hospital Structures where they operate, with the necessary involvement of the concerned Hospital Authority and subject to obtaining the favorable opinion of the competent Ethics Committee.
The agreement generally involves compensation for the University, and the contract is of a remunerative nature.
For further information regarding procedures and regulatory references, please contact:
Legal and General Affairs Office - This email address is being protected from spambots. You need JavaScript enabled to view it.